MEDIUM
Earn 100
Which neighbouring country is located to the east of India?
(a)M,y,a,n,m,a,r
(b)P,a,k,i,s,t,a,n
(c)A,f,g,h,a,n,i,s,t,a,n
(d)M,a,l,d,i,v,e,s
50% studentsanswered this correctly
Important Questions on Relations and Functions
HARD
Let be a polynomial of degree four and having its extreme values at and . If , then is equal to
EASY
EASY
EASY
EASY
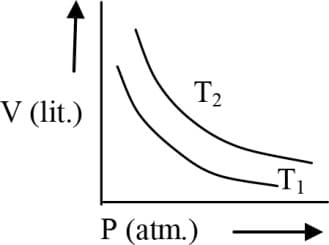
EASY
One mole of an ideal gas at is expanded isothermally from an initial volume of litre to litre. for this process is:
EASY
MEDIUM
EASY
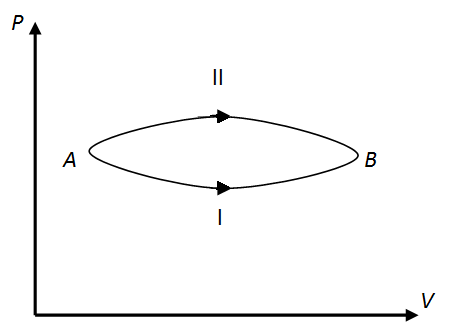
MEDIUM
EASY
The pressure (P) and density of a diatomic gas changes from to . What is the value of if
EASY
EASY
EASY

