EASY
JEE Main
IMPORTANT
Earn 100
Which of the following are characteristics of physisorption?
a. Weak attractive interactions between adsorbate and adsorbent
b. Monolayer formation of adsorbent on adsorbate
c. Remains unaffected by change of temperature
d. Decreases with increase of temperature
e. It is also known as activated adsorption
69.57% studentsanswered this correctly
Important Questions on Surface Chemistry
MEDIUM
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
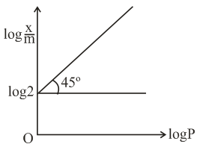
Graph between and is a straight line at an angle with intercept is shown above at a pressure of is:
MEDIUM
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
The catalyst iron employed in the Haber's process contains molybdenum, the function of which is
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
