Which of the following is/are correct about ?

Important Questions on Solid State
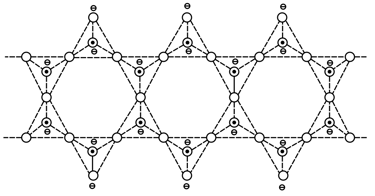
Which mineral can have the silicates skeleton as shown below?
Copper has a face-centred cubic structure with a unit-cell edge length of . What is the size of the largest atom (in pm), which could fit into the interstices of the copper lattice without distorting it?
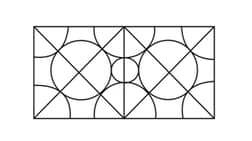
[Hint: Calculate the radius of the smallest circle in the figure.]
A metal cystallises into two cubic phases, f.c.c and b.c.c. whose unit-cell lengths are and . Calculate the ratio of densities of f.c.c. and b.c.c.
Give answer after multiplying with 100 and rounding off to the nearest integer value.
Potassium metal crystallizes in a face-centred arrangement of atoms where the edge of the unit cell is . Determine the shortest separation of any two potassium nuclei in pm is.
Sodium metal crystallizes in a body-centred cubic lattice with the cell edge . What is the radius of the sodium atom in pm? (Round off your answer to the nearest integer value.)
A metallic element has a simple cubic lattice. Each edge of the unit cell is The density of the metal is How many unit cells will be present in of the metal?
(Give your answer in multiples of .)
