MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following plots is correct for solutions of different solutes having the same density?
is molarity and is molar mass of the solute.
(a)

(b)
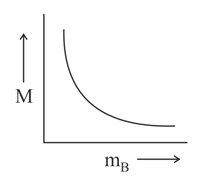
(c)
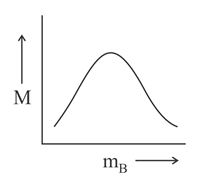
(d)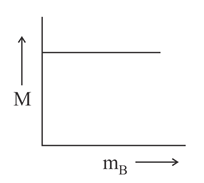

42.11% studentsanswered this correctly

Important Questions on Solutions
EASY
JEE Main
IMPORTANT
The molarity of a solution obtained by mixing of with of will be:
MEDIUM
JEE Main
IMPORTANT
The vapour pressure of a dilute solution of a solute is influenced by
MEDIUM
JEE Main
IMPORTANT
By adding water to the solution of an ionic compound, its
HARD
JEE Main
IMPORTANT
For a binary ideal liquid solution, the variation in total vapuor pressure versus composition of solution is given by which of the curves?
MEDIUM
JEE Main
IMPORTANT
solution and solution have same
HARD
JEE Main
IMPORTANT
Which of the following conversion is/are correctly depicted? The symbols used are molality of the solution; molarity of the solution; and mole fractions of solvent and solute respectively. and molar masses of solvent and solute respectively, density of solution. and are amounts of the solvent and solute respectively.
MEDIUM
JEE Main
IMPORTANT
Components of a binary mixture of two liquids and , were being separated by distillation. After some time, the separation of the components stopped and the composition of the vapour phase became the same as that of the liquid phase. Both the components started appearing in the distillate. Explain why did this happen?
MEDIUM
JEE Main
IMPORTANT
Cyclohexane and ethanol at a particular temperature have vapour pressures of and , respectively. If these two solutions, having mole fraction value of cyclohexane equal to , are mixed and the mixture has a total vapour pressure of will the mixture be an ideal solution?
