EASY
Earn 100
Which statement is true?
(a)Resonance hybrids are inherently unstable
(b)Resonance hybrids are more stable than any individual resonance form
(c)Resonance hybrids are averages of all resonance forms resembling the less stable forms
(d)Resonance hybrids are averages of all resonance forms resembling the more stable forms
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
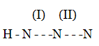
The above compound represents hydrogen azide, the bond orders of bonds and are:
EASY
EASY
EASY
MEDIUM
[Resonance energy of benzene
Enthalpy of hydrogenation of cyclohexene ]
MEDIUM
Resonance in carbonate ion is
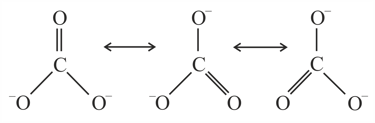
Which of the following is true?
MEDIUM
Give a reason for the following:
Ionic compounds have a high melting point.
MEDIUM
The molecule/molecules that has/have delocalised lone pair(s) of electrons is/are
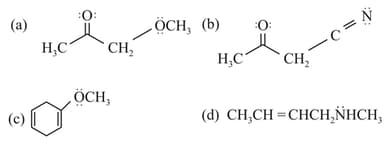
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM

