Resonance
Resonance: Overview
This topic covers concepts, such as Conditions for Resonance, Resonance, Resonance Hybrid, Rules for Writing Canonical Forms, Identification of the Most Stable Resonating Structures, Calculation of Bond Order Using Resonating Structures, etc.
Important Questions on Resonance
For phenol, which of the following resonating structure is the most stable?
is more stable than because
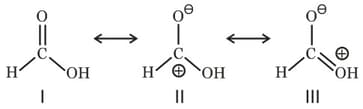
Among these canonical structures, the correct order of stability is
Among, these, which are canonical structures?
Which species has the bond order is equal to
Resonance structures are not considered isomers. Why?
Which statement is true?
Dispel some misconceptions associated with resonance?
Write the difference between Resonance and isomerism.
Which of the following resonating structure is most stable?
Write the following statement of about RESONANCE are true or false.
There is no equilibrium between the canonical forms.
Write the following statement of about RESONANCE are true or false.
The molecule does exist for a certain fraction of time in one canonical form and for other fractions of time in other canonical forms.
State if the following statements about RESONANCE are true or false.
Canonical forms of any structure have no real existence.
Assertion. The resonance hybrid is more stable than any of the contributing structure.
Reason. The contributing structures contain the same number of unpaired electrons and have real existence.
Consider the following resonating structure. What will be the correct decreasing order of stability?
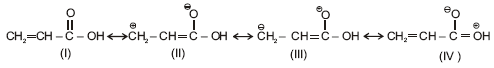
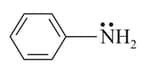
Which of the given options represents the correct order of bond length in the following amines?
Which of the following statements are correct for butadiene
(1) The and bonds are longer than a carbon-carbon double bond.
(2) The and bonds are shorter than a carbon-carbon double bond.
(3) The bond is slightly shorter than a carbon-carbon single bond.
(4) The bond is slightly longer than a carbon-carbon single bond.
The correct order of bond lengths in is:
Which of the following molecules does not show any resonating structures?
The nitrite ion in represented by
(I)
(II)
Which of the structures represents possible resonance forms of this ion?
