MEDIUM
9th CBSE
IMPORTANT
Earn 100
Why do helium, neon and argon have a zero valency?

Important Questions on Structure of Atom
HARD
9th CBSE
IMPORTANT
1. , and are the isotopes of neon.
(i) What do the subscripts and superscripts represent?
(ii) What is the cause of change in superscripts?
(iii) Give the geometric diagram of .
HARD
9th CBSE
IMPORTANT
What information do you get from the figure given about the atomic number, mass number and valency of atoms X, Y, and Z?. Give your answer in a tabular form.
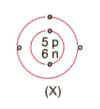
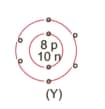
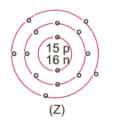
MEDIUM
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
The average atomic mass of a sample of an element X is 23.04 m. What are the percentage of isotopes in the sample?
MEDIUM
9th CBSE
IMPORTANT
EASY
9th CBSE
IMPORTANT
EASY
9th CBSE
IMPORTANT
EASY
9th CBSE
IMPORTANT
