EASY
Earn 100
Why drastic conditions are required for converting chlorobenzene to phenol although conversion of dinitrochlorobenzene into dinitrophenol is very easy?
(a)
makes the electron rich ring at ortho and para-positions.
(b) withdraws electrons at meta position.
(c) donates electrons at meta position.
(d) withdraws electrons from ortho- and
para-position
para-position
80% studentsanswered this correctly
Important Questions on Alcohols,Phenols and Ethers
HARD
MEDIUM
HARD
HARD
HARD
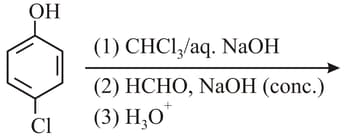
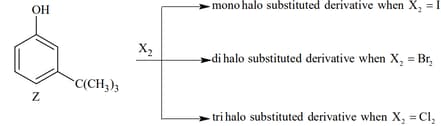
In the following reaction, the product(s) formed is/are:
HARD
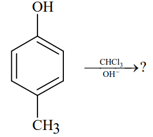
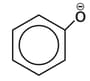
The observed pattern of electrophilic substitution can be explained by
EASY
 ion ?
ion ?EASY
Match the following values.
| Acid | |||
| (a) | Phenol | (i) | |
| (b) | -Nitrophenol | (ii) | |
| (c) | Ethanol | (iii) | |
| (d) | Picric acid | (iv) |
HARD
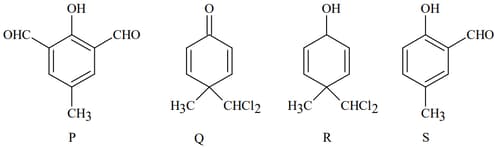
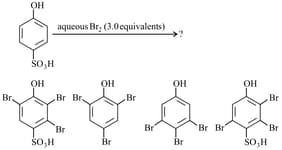
The major product(s) of the following reaction is/are:
MEDIUM
MEDIUM
| Test | Inference | |
|---|---|---|
| Insoluble | ||
| Soluble | ||
| Decolourization |
HARD
The major product of the following reaction is:
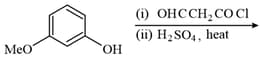
MEDIUM
HARD
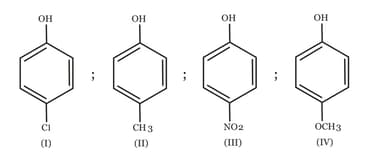
The increasing order of the values of the following compounds is:
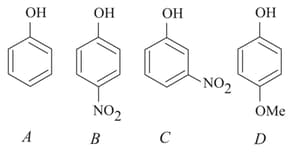
MEDIUM
MEDIUM
HARD
HARD
What is the product "C" after following reactions -
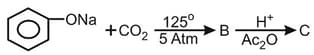
MEDIUM
MEDIUM