EASY
JEE Main
IMPORTANT
Earn 100
With respect to graphite and diamond, which of the following statement(s) given below is/are correct?
(a)Graphite is harder than diamond.
(b)Graphite has higher electrical conductivity than diamond.
(c)Graphite has higher thermal conductivity than diamond.
(d)Graphite has higher bond order than diamond.
12.5% studentsanswered this correctly

Important Questions on Solid State
EASY
JEE Main
IMPORTANT
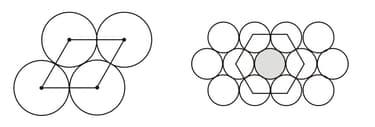
What is the relation between the radius of circle and the length of parallelogram for the unit cell shown in figure below?
MEDIUM
JEE Main
IMPORTANT
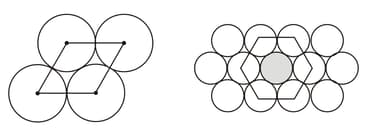
How many nearest neighbor circles does a given circle have in the second figure below?
MEDIUM
JEE Main
IMPORTANT
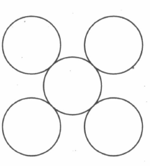
What is the packing efficiency of the two-dimensional square unit cell shown below?
HARD
JEE Main
IMPORTANT
Xenon crystallises in the face-centred cubic lattice and the edge of the unit cell is . What is the nearest neighbour distance and what is the radius of Xenon atom?
HARD
JEE Main
IMPORTANT
Calculate the approximate number of unit cells present in of gold. Given that gold crystallizes in the face-centred cubic lattice (Atomic mass of gold ).
EASY
JEE Main
IMPORTANT
The intermetallic compound has a cubic crystalline structure in which each atom has eight nearest neighbor silver atoms and vice-versa. What is the type of unit cell?
MEDIUM
JEE Main
IMPORTANT
A compound alloy of gold and crystallizes in a cubic lattice in which the gold atoms occupy the lattice points at the corners of a cube and the copper atoms occupy the centres of each of the cube faces. What is the empirical formula of this compound?
EASY
JEE Main
IMPORTANT
How many 'nearest' and 'next nearest' neighbors respectively potassium has in lattice?
