MEDIUM
10th CBSE
IMPORTANT
Earn 100
Write a balanced chemical equation and identify the type of chemical reaction taking place when:
Zinc metal reacts with aqueous hydrochloric acid to produce a solution of zinc chloride and hydrogen gas.

Important Questions on Chemical Reactions and Equations
EASY
10th CBSE
IMPORTANT
Iron nails when left dipped in blue copper sulphate solution become brownish in colour and blue colour of copper sulphate fades away.
MEDIUM
10th CBSE
IMPORTANT
Quick lime reacts vigorously with water releasing a large amount of heat.
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
MEDIUM
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
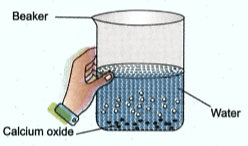
Solid calcium oxide was taken in a container and water was added slowly to it.
State the two observations made in the experiment.
EASY
10th CBSE
IMPORTANT
Solid calcium oxide was taken in a container and water was added slowly to it.
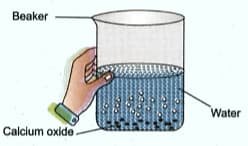
Write the name and the chemical formula of the product formed.
