EASY
Earn 100
Write the effect of the molar mass on Maxwell-Boltzmann's distribution curve of molecular speeds.
Important Questions on States of Matter: Gases and Liquids
EASY
MEDIUM
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
Select from the box, the correct gas law related to the given situation.
| Boyle's law, Charles law, Avagadro's law |
An inflated balloon kept at sunlight bursts after some time.
MEDIUM
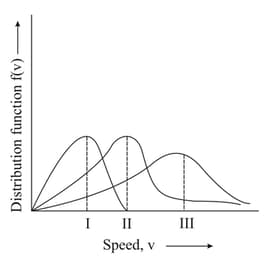
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
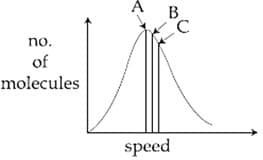
Root mean square speed most proable speed Average speed
MEDIUM

