EASY
Earn 100
Write the formula to calculate the specific rotation of an optically active compound.
Important Questions on Organic Chemistry (AHL)
HARD
For the given compound X, the total number of optically active stereoisomers is ____.
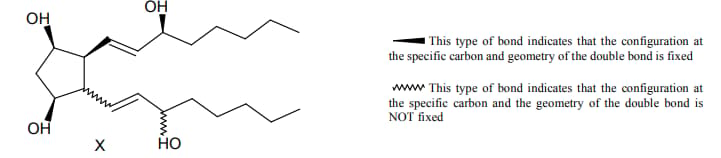
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
I. Trans-1-chloro-2-methylcyclopropane
II. Cis-1-chloro-2-methylcyclopropane
III. 1-chloro-1-methylcyclopropane
IV. Cis-1, 2-dichlorocyclopropane
HARD
EASY
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
MEDIUM
EASY
HARD
Trans-3,6-Dimethyl oct-4-ene (A) exists in two diastereomers and .
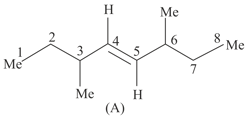
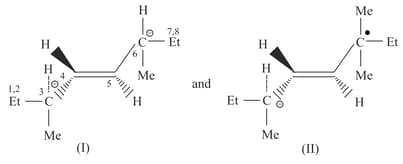
Which statements is true about and ?
MEDIUM
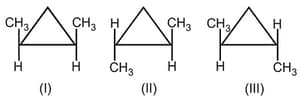
HARD
HARD
MEDIUM
(+) - mandelic acid has a specific rotation of 158o. What would be the observed specific rotation of a mixture of 25% (-) - mandelic acid and 75% (+) - mandelic acid ?
MEDIUM


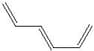 the double bonds are
the double bonds are