Zinc and lead are obtained from ores that contain only the metal and sulfur, in the molar ratio 1:1.
Write down the formulae of their compounds.

Important Questions on Making Use of Metals
Zinc and lead are obtained from ores that contain only the metal and sulphur in the molar ratio . In the extraction of the metal, the compounds are roasted in the air to obtain the oxide of the metal. The sulphur forms sulphur dioxide.
Write equations for the roasting of the ores.
Zinc and lead are obtained from ores that contain only the metal and sulphur, in the molar ratio 1:1. In the extraction of the metal, the compounds are roasted in air to obtain the oxide of the metal. The sulphur forms sulphur dioxide.
Which type of reaction is this?
Zinc and lead are obtained from ores that contain only the metal and sulphur, in the molar ratio 1:1. In the extraction of the metal, the compounds are roasted in air to obtain the oxide of the metal. The sulphur forms sulphur dioxide.
Care must be taken in disposing of the sulphur dioxide produced. Explain why?
Zinc and lead are obtained from ores that contain only the metal and sulphur, in the molar ratio 1:1. In the extraction of the metal, the compounds are roasted in air to obtain the oxide of the metal. The sulphur forms sulphur dioxide. Then the oxide can be heated with coke (carbon) to obtain the metal and carbon monoxide.Write equations for the reactions with carbon.
Zinc and lead are obtained from ores that contain only the metal and sulphur, in the molar ratio 1:1. In the extraction of the metal, the compounds are roasted in air to obtain the oxide of the metal. The sulphur forms sulphur dioxide. Then the oxide can be heated with coke (carbon) to obtain the metal and carbon monoxide. Which substances are reduced in the reaction?
Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The table below summarises the two extraction processes. In each extraction, is the metal oxide oxidised or reduced, when it is converted to the metal?
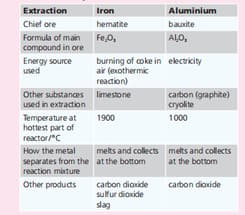
Aluminium is the most abundant metal in the Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The table below summarises the two extraction processes. Explain why each of these substances is used: limestone in the extraction of iron.
| Extraction | Iron | Aluminium |
| Chief ore | hematite | bauxite |
| Formula of main compound in ore | ||
| Energy source used | burning of coke in air(exothermic reaction) | Electricity |
| Other substances used in extraction | limestone | carbon(graphite), cryolite |
| Temperature at hottest part of reaction/ | 1900 | 1000 |
| How the metal separates from the reaction mixture | melts and collects at the bottom | melts and collects at the bottom |
| Other products | Carbon dioxide, sulphur dioxide, slag | Carbon dioxide |
Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The table below summarises the two extraction processes. Explain why each of these substances is used: carbon in the extraction of aluminium.
| Extraction | Iron | Aluminium |
| Chief ore | hematite | bauxite |
| Formula of main compound in ore | ||
| Energy source used | burning of coke in air(exothermic reaction) | Electricity |
| Other substances used in extraction | limestone | carbon(graphite), cryolite |
| Temperature at hottest part of reaction/ | 1900 | 1000 |
| How the metal separates from the reaction mixture | melts and collects at the bottom | melts and collects at the bottom |
| Other products | Carbon dioxide, sulphur dioxide, slag | Carbon dioxide |
