Electronic Effects
Important Questions on Electronic Effects
Most stable and least stable species respectively among the following are
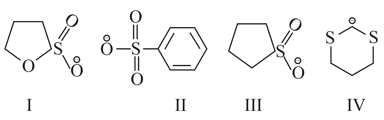
The correct stability order of the following resonance structure is
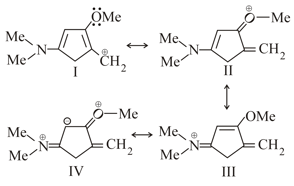
Number of hyperconjugate hydrogen of propene is
In which of the following molecules positive charge is not delocalized because of resonance?
Resonance is not possible in
Which of the following group will have strongest electron donating mesomeric effect?
In the given anion, charge is delocalized on
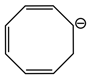
At conjugated position imparts
In which of the following all electronic effects namely inductive, mesomeric and hyperconjugative effects are present?
Arrange following three given aromatic compounds in increasing order of resonance energy per ring.
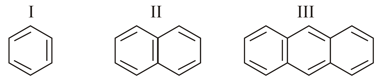
Which of the following is most stabilised by hyperconjugation?
In which of the following alkene hyperconjugation does not take place?
Which of the following pairs are resonance structures?
I. and 
II. 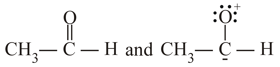
III. 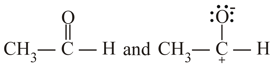
IV. and
The most stable resonating structure of methoxyethene is
Among the following three canonical structures what would be their relative contribution in hybrid?
I.
II.
III.
Given set of resonating structures and their stability is provided in bracket. Select which one is incorrectly matched.
In which of the following species lone pair at Nitrogen atom is not involved in delocalization?
Choose the correcct statement.
The inductive effects of the groups 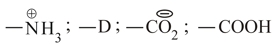
are respectively
The mesomeric effects of the groups
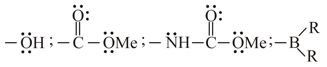
are respectively.

