Bond Parameters
Bond Parameters: Overview
This topic covers concepts such as bond parameters, bond length, bond enthalpy, bond order and crystal lattice formation of nacl.
Important Questions on Bond Parameters
Bond formation is :
In the compound,  , the bond is of the type –
, the bond is of the type –
How many among the given species have a bond order of
Arrange the following molecules according to decreasing order of their bond lengths.
Which pair among the following does not have the same bond order?
The correct order of decreasing bond angle is :
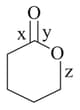 Correct order of Bond length is:
Correct order of Bond length is:
Peroxy linkage is present in :-
Among the following species, which has the maximum bond order
Assertion. molecule is more stable than molecule.
Reason. The anti-bonding electron in the molecule destabilises it.
Statement-I. molecule is more stable than molecule.
Statement-2. The antibonding electron in the molecule destabilises it.
Maximum bond distance can be observed in which of the following?
In which of the molecule, bond is found to be strongest?
Match the bond enthalpies given in , in column with the given molecules in column and choose the appropriate answer:
| Column - I | Column - II | ||
|---|---|---|---|
| Hydrogen | |||
| Oxygen | |||
| Nitrogen |
Ionic resonance energy of the bonds are given as follows,based on the given information which bond is the most polar bond?
Bonds Ionic resonance energy
24.3
50.6
102.3
105.9
Among the following processes, in which process the bond order has increased and paramagnetic character has changed to diamagnetic?
Which one displays the correct order of bond energy?
What is the correct order of bond angle of the following species?
In a compound AB, atomic radius A and B are and . Electronegativity difference between A and B is 1.9. The distance between A and B atoms, is:
Shortest bond can be seen in which of the following?
