Hybridisation
Hybridisation: Overview
This topic covers concepts such as Rules for Hybridisation, sp3d Hybridisation, sp3d2 and d2sp3 Hybridisations, dsp2 Hybridisation & Hybrid Orbitals and Salient Features of Hybridisation.
Important Questions on Hybridisation
The hybridisations of and shown in the following compound
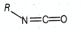
respectively, are
The hybridisation of xenon atom in is
Which of the following is the correct order regarding the electronegativity of hybrid orbitals of carbon?
In an octahedral structure, the pair of d-orbitals involved in hybridisation is
Assertion. does not have a tetrahedral structure.
Reason. in has two lone pairs
Find the correct match for the column , from the column and choose the correct option:
| Column - I | Column - II | ||
|---|---|---|---|
| hybridisation | |||
| hybridisation | |||
| hybridisation | |||
| hybridisation |
The geometry and hybridisation of is
The number of peroxide bonds in perxenate ion is:
Given below is the bond angle in various types of hybridisation. Mark the bond angle which is not correctly matched.
Hybridisation involves
Which of the following is an octahedral molecule?
The molecule has a linear structure. This compound has
Which of the following molecules has the maximum number of atoms in a plane?
In which of the following species only one type of hybridization is present?
Number of bonding electron pairs and number of lone pairs of electrons in respectively are
Which one of the following molecules will have unequal M - F bond lengths?
Linear combination of two hybridized orbitals belonging to two atoms and each having one electron leads to a
Hybridization of central I in is.
Decreasing order of size of various hybrid orbitals is -
In general, the d-orbital involved in d hybridisation is
