Octet Rule and its Limitations
Octet Rule and its Limitations: Overview
This topic covers concepts, such as, Atom, Chemical Bonds, Lewis Structure for Simple Molecules & Electron Deficient Species etc.
Important Questions on Octet Rule and its Limitations
According to the Lewis formula of , the correct option is
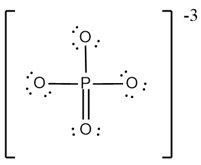
What are the formal charges on and in the Lewis (electron dot) structure of the phosphate oxyanion represented in the figure?
Electron deficient species among the following is:
List some odd electron molecules.
Which of the following oxides is an odd-electron molecule.
Which among the following is least covalent in nature?
Why noble gases do not form compounds except krypton, xenon, and radon?
Which of the following noble gas compound has hybridisation?
What are the noble gases which form compounds?
If an element is represented by its Lewis symbols as 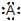 , then A may be
, then A may be
What will be the probable chemical formula if the valence shell of element contain three electrons, while the valence shell of element contain six electrons are combined?
In the Lewis structure, the formal charge on the central atom of is
Maximum number of lone pairs is present in Lewis dot structure of which compound?
How are sodium and chlorine atoms attain octet configuration in the formation of sodium chloride.
What are valence electrons? Write the number of valence electrons in the chlorine and carbon.
Find the odd- electron molecules from the following
(a)
(b)
(c)
(d)
(e)
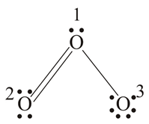
In ion formal charge on the oxygen atom of bond is
The formal charge on the -atoms in the ion is
In which of the following molecule central atom is having complete octet?
