Valence Bond Theory-Orbital Overlap Concept
Valence Bond Theory-Orbital Overlap Concept: Overview
This topic covers concepts, such as, Valence Bond Theory, Comparison of Sigma and Pi Bonds, Lateral Overlapping & Axial Overlapping etc.
Important Questions on Valence Bond Theory-Orbital Overlap Concept
In which of the following molecules both phenyl rings are not coplanar ?
Number of sigma bonds in is :
The correct statement for the structure of is
Which among the following represents zero overlap?
Which of the following has bonding?
Propyne molecule contains sigma and _____ pi bonds.
The sum of number of sigma and pi bonds formed between two carbon atoms in are:
Select the correct match : (consider axis as internuclear axis)
Degree of unsaturation for given compound will be:
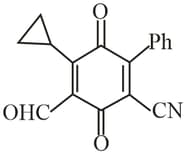
Geometry of which of the following can't be explained using (pure overlapping)
In which of the following species central atom forms compound in excited state, if excitation is considered to be true ?
Which of the following overlapping results in formation of -bond, if is taken as internuclear axis ?
sigma and bond in given molecule is:-
Assuming all non-hydrogen atoms undergo hybridization & following are various categories of orbital overlapping.
axial overlap Type
axial overlap Type
axial overlap Type
lateral ovelap Type
lateral overlap Type
Identify the species which contain only of these types (from to ) of overlapping of orbitals.
The no. of and bonds are present in given compound.
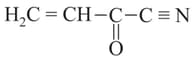
Which of the following combinations of atomic orbitals can form only one type of covalent bond (out of bond) :-
Correct order of extent of axial overlapping is :-
Number and type of bonds between two carbon atoms in CaC2 are
Which of the following is not tetrahedral in shape?
The number of and bonds in allyl isocyanide are
