Periodic Law and the Modern Periodic Table
Periodic Law and the Modern Periodic Table: Overview
This topic covers concepts, such as, Modern Periodic Table, Modern Periodic Law, Atomic Number for Arranging Elements & Moseley's Experiment etc.
Important Questions on Periodic Law and the Modern Periodic Table
Assertion (A) : Sixth period is the longest period in the periodic table.
Reason (R) : Sixth period involves the filling of all the orbitals of the sixth energy level.
The correct order of filling up of subshells in the seventh period is
Which of the following groups does helium belongs?
All of the group elements have valence shell electronic configuration and the properties of an element have
Which of the following statement is incorrect?
transition series of elements start with scandium which has the electronic configuration
Successive filling of and orbitals give rise to the third period. The number of elements present in this period are
An element belongs to third period and fifteenth group of the periodic table. Which one of the following is true regarding the outer electronic configuration of ?
An element has its electronic configuration as 2, 8, 2. What is the group of this element?
Henry Moseley plotted a graph between and , where was the frequency of ray and was its atomic number.This graph showed that
Periodic classification of elements can be done on the basis of electronic configuration and is used to examine the
Maximum possible number of elements present in period
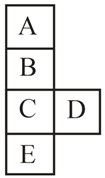
Then choose the correct statement:
Atoms of the elements belonging to the same group of periodic table will have
Which statement is wrong for the long form of periodic table:-
The electronic configuration of an element is . What is the atomic number of next element of the same group which is recently discovered?
In short period, number of elements are:
Among the following, Which is not correct statement for periodic classification of elements ?
In which block the non-metals are placed in the long form of periodic table?
Writr the name and symbol of the element with atomic number is :
