Reduction of Metal Oxides to Metals
Reduction of Metal Oxides to Metals: Overview
This topic covers concepts such as Self-Reduction Process, Smelting, Reduction of Metals by Hydrogen, and Pyrometallurgy.
Important Questions on Reduction of Metal Oxides to Metals
Copper can be extracted from
Copper can be extracted from
The reducing agent used for the extraction of and is _____.
(A)
(B)
(C)
Enter your correct answer as A, B or C.
Discuss the reduction of metal by hydrogen.
The process which involves smelting is
Which of the following reactions is an example of autoreduction?
Which are the different methods used in metallurgy?
What is smelting? Explain with an example.
Explain the term:
Smelting.
What is cryolite?
Metal that cannot be extracted by carbon reduction process is:
In the bessemer converter which is used for the extraction of copper, select the correct option(s):
In self-reduction, the reducing species is
Identify
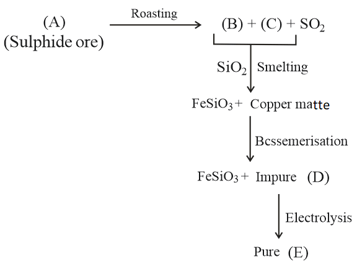
Identify
Coke powder is spread over molten electrolyte to:
The chemical composition of the slag formed during smelting is:
Which is known as 'blister copper' ?
carbon reduction is used for the extraction of:
Which of the following reactions show the process of smelting?
