Systematic Analysis of Cationic Radicals
Systematic Analysis of Cationic Radicals: Overview
This topic covers concepts, such as, Sodium Hydroxide Solution Test for Ammonium Ion, Ammonia Solution Test for Copper(II) Ion, 4-(p-Nitrophenylazo)resorcinol Solution Test for Magnesium Ion & Titan Yellow Test for Magnesium(II) Ion etc.
Important Questions on Systematic Analysis of Cationic Radicals
The reaction of and in water produces a precipitate that dissolves upon the addition of of appropriate concentration. The dissolution of the precipitate is due to the formation of
Find the number of compounds which have yellow colour precipitate from the given compounds:
A reagent used to test nickel ion is ______.
A colourless water soluble solid on heating gives equimolar quantities of and . gives dense white fumes with and does so with gives brown precipitate with Nessler's reagent and gives white precipitate with nitrates of and is:
reacts with excess of solution to produce which of the folloing?
Which of the following are produced when reacts with excess of ammonia solution?
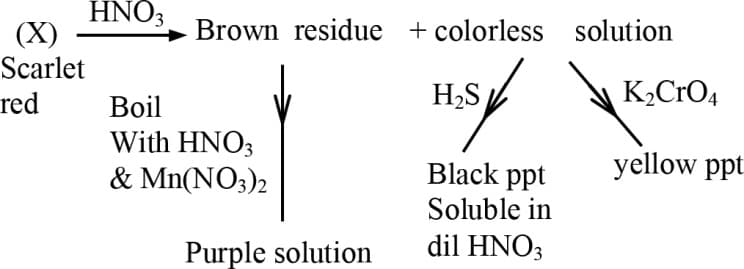
Therefore is -
Name the metal which reacts with sodium hydroxide solution, liberating hydrogen gas.
A white gelatinous precipitate is formed when this compound reacts with sodium hydroxide.
The pair of ions that can not be separated by in the presence of dilute hydrochloric acid are
When ammonia gas is passed through Nessler's solution the colour becomes-
Which of the following sulphide would dissolve in hot and concentrated nitric acid?
On adding sodium hydroxide drop by drop to a solution of ferrous sulphate, a dirty green precipitate is formed. Write the chemical formula of the compound formed of this colour.
(Enter your correct answer as )
The colour of precipitate formed when sodium hydroxide is added to a solution of ferric chloride is _____.
The addition of an excess of ammonium chloride ammonia buffer to a solution containing nitrates of and will precipitate hydroxides of
A white gelatinous precipitate is formed when this compound reacts with sodium hydroxide.
is obtained from
Read the following sequence of reactions:
What are the compounds and ?
The coordination number of Hg in Millon's base is
When aqueous solution of copper sulphate is added in excess of dilute , an intense blue colour is obtained. This is due to formation of-
