Position of d-Block and f-Block Elements in Periodic Table
Position of d-Block and f-Block Elements in Periodic Table: Overview
This topic covers concepts, such as The d- and f-Block Elements, d-Block Elements, Transition Metals, Position of d-Block Elements in the Periodic Table, Four Series of d-Block Elements, and f-Block Elements.
Important Questions on Position of d-Block and f-Block Elements in Periodic Table
On what grounds can you say that scandium is a transition element but zinc is not one?
Which of the following statements are true.
(i) do not show any colour in solutions.
(ii) Among the divalent cations in the first series of transition elements, manganese exhibits the maximum paramagnetism.
The steady decreases in the ionic radius from to is termed as
Using the Hund’s rule the electronic configuration of ion and its magnetic moment by using ‘spin only’ formula would be:
Select the correct statement among the following :-
Which of the following is not a transition element?
Consider the given diagram
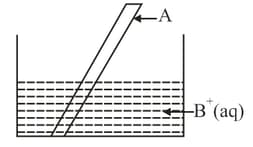
If on dipping in , the colour of the solution changes from colourless to blue then and respectively are
Which pair of lanthanides is used in glass, blowers, goggles ?
Which of the following group of block does not contain transition element?
-block elements are also known as
In -block elements, the differentiating electron enters into
Which of the following statement is incorrect for pair of elements ?
Which element doesnot belong to transition metal.
The increase in atomic number with reduction in atomic size is a general characteristic of elements of -
The true statement for an element with atomic number is :
The general electronic configuration of transition element is
The group which belongs to transition series is
Transition metals have high melting points, mainly because of
The general oxidation states shown by Ce (cerium) in its compounds are:
Among the following, which one lanthanide ion does not exhibit paramagnetism?
