General Introduction of Group 13 Elements
General Introduction of Group 13 Elements: Overview
This topic covers concepts, such as, Group 13: The Boron Family, Occurrence of Group 13 Elements, Ionisation Enthalpy of Group 13 Elements & Electronegativity of Group 13 Elements etc.
Important Questions on General Introduction of Group 13 Elements
For compound having the formula , the correct option from the following is
Oscillating trend of ionization energy is found in:
The characteristic valence shell configurations of group- and elements are respectively
What is the correct form of the electronic configuration of thallium element?
(i) (Order of )
(ii) (Order of )
(iii) (Order of radius)
(iv) (Order of EN)
Select correct order:
Incorrect statement about diborane is:
In following reactions how many boron products are produced?
$\mathrm{BF}_{3}+\mathrm{LiAlH}_{4} \stackrel{\text { ether }}{\longrightarrow} \mathrm{A}+\mathrm{B}+\mathrm{C}$
$\mathrm{BF}_{3}+\mathrm{NaH} \stackrel{450 \mathrm{K}}{\longrightarrow} \mathrm{D}+\mathrm{E}$
$\mathrm{Na}_{2} \mathrm{B}_{4} \mathrm{O}_{7}+7 \mathrm{H}_{2} \mathrm{O} \longrightarrow \mathrm{F}+\mathrm{G}$
Diborane is obtained by the oxidation of sodium borohydride with iodine. If 10 moles of sodium borohydride is reacted with iodine then how many moles of diborane will be produced ?
In diborane, how many hydrogen atoms account for bonding in the bridges?
The outer electronic configuration of group is given as , where, value of is
Structure of diborane: Give the correct statement:
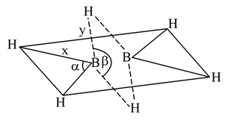
The number of linkage present in the pentaborate anion of Borax is:
Which of the following is correct for the structure of diborane?
Match List I with List II
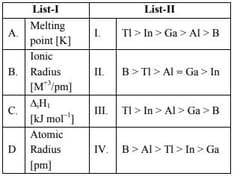
Choose the correct answer from the options given
below :
Given below are two statements :
Statement I: In group 13, the stability of +1 oxidation state increases down the group.
Statement II: The atomic size of gallium is greater than that of aluminium.
In the light of the above statements, choose the most appropriate answer from the options given below:
The correct order of the first ionization enthalpy is
Correct order of increasing atomic size of group element will be:
Assertion: For the group, the oxidation state increases down the group.
Reason: The atomic size of is greater than that of .
When Diborane reacts with and independently, then products formed are:
Which one of the following correctly represents the variation of electronegativity with atomic number () of group 13 elements?
