Nitric Acid: Preparation, Structure, Properties and Uses
Nitric Acid: Preparation, Structure, Properties and Uses: Overview
This topic covers concepts, such as Nitric Acid, Preparation of Nitric Acid, Manufacture of Nitric Acid by Ostwald's Process, Structure of Nitric Acid, Physical Properties of Nitric Acid, and Chemical Properties of Nitric Acid.
Important Questions on Nitric Acid: Preparation, Structure, Properties and Uses
All of the given statements are true for nitric acid except
In brown ring test for nitrate ions, brown ring is formed having composition
Metal which become passive with conc. is
on disproportionation gives and
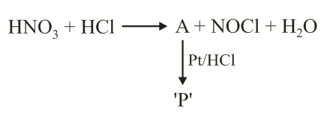
The product will be
and respectively are
In brown ring test for nitrate ions, brown ring is formed having composition
Concentrated nitric acid turns the colour of the skin into-
The brown ring test for nitrates depends upon
When reacts with concentrated , the gas evolved is
The oxidation state of nitrogen in is:
When the copper metal is treated with a cold and dilute nitric acid, it forms
Which of the following does not produce gas with conc. ?
Select the correct statement from the following:
When copper is heated with conc. it produces
In the brown ring test, brown colour of the ring is due to
A brown ring is formed in the ring test for ion. It is due to the formation of
Select the correct statement.
forms brown ring with
In which of the following reactions, reaction of silver with the given acids lead to the formation of compound & silver, along with liberation of gas ?
