Potassium Dichromate
Potassium Dichromate: Overview
This topic sheds light on potassium dichromate, its preparation and structure, along with its reactions in alkaline and acidic medium. It also highlights the physical and chemical properties of potassium dichromate and its uses.
Important Questions on Potassium Dichromate
Write the net ionic equation for the reaction of potassium dichromate with sodium sulphite in an acid solution to give chromium ion and the sulphate ion.
The following steps are involved in the manufacturing of potassium dichromate:
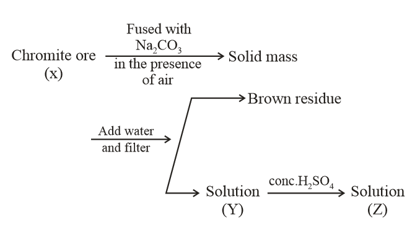
What is the difference in the oxidation number of between X and Y?
What is the colour change when solid potassium dichromate is heated with concentrated sulphuric acid?
Solid potassium dichromate when heated with concentrated sulphuric acid gives which of the following product?
When potassium dichromate reacts with hot sulphuric acid, violet fumes are formed.
Write the reaction of potassium dichromate with hot concentrated sulphuric acid.
when reacts with cold conc. gives red crystal of
Which of the following product is not formed when potassium dichromate reacts with cold sulphuric acid?
When potassium dichromate reacts with cold sulphuric acid, the red crystals formed are of .
Write the balanced equation of the reaction of oxidising Nitrite ion to Nitrate in the presence of acidified potassium dichromate.
When sodium nitrite reacts with acidified Potassium Dichromate, what is produced?
When acidified Potassium Dichromate reacts with Nitrite ion, what will happen?
How is potassium dichromate prepared from chrome iron?
If turns acidified solution green, then may be:
How many moles of are needed to oxidise a mixture of completely in an acidic medium?
When a mixture of solid solid is heated with conc. orange red vapours are obtained of the compound
Which of the following can give two or more gases on heating?
Regarding the solution of which of the following is/are correct ?
To an acidified dichromate solution, a pinch of is added and shaken. What is observed?
One of the product formed when reacts with conc. in cold is
