Esters
Important Questions on Esters
The product of acid hydrolysis of and can be distinguished by which of the following:
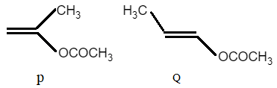
An ester with molecular formula was treated with an excess of and the complex so formed was treated with to give an olefin Ozonolysis of gave a ketone with molecular formula which shows iodoform test. The structure of is:
Which of the following esters cannot undergo Claisen self-condensation?
Which one of the following esters cannot undergo Claisen self-condensation?
An ester is boiled with The product is cooled and acidified with concentrated A white crystalline acid separates. The ester is:
When esters are hydrolysed, the product gives hydrogen ions. The product which gives hydrogen ion is:
Self-condensation of two moles of ethyl acetate in the presence of sodiumethoxide after acidification yields:
Hydrolysis of an ester gives a carboxylic acid, which on Kolbe's electrolysis, yields ethane. The ester is:
The hydrolsis of an ester gives a carboxylic acid, which on Kolbe's electrolysis yields ethane. The ester is
An ester is boiled with . When the product is cooled and acidified with concentrated , a white crystalline acid separates. The ester is
What is the following reaction called?
Hydrolytic reaction of fats with caustic soda is known as
Methyl acetate will be obtained by reacting with

