Effect of Temperature on Equilibrium Constant
Important Questions on Effect of Temperature on Equilibrium Constant
Which of the following curves between and is correct for an endothermic reaction?
The energy profile of the reaction, is shown as,
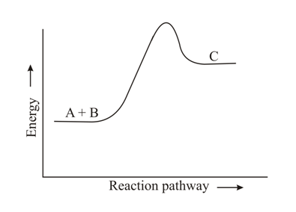
The equilibrium constant for the said equilibrium
The equilibrium constants for the reaction at and are and , respectively. The reaction is
For the gas phase reaction , carried out in a vessel, the equilibrium concentration of can be increased by
From the given data at , calculate the equilibrium constant at for
.
Find the value of (Nearest integer).
Equilibrium constants for the reaction are and at and respectively. Calculate the heat of formation of gaseous ammonia.
Equilibrium constant for the reaction, is at and the heat of dissociation is . Find equilibrium constant .
Would you expect the equilibrium constant for the reaction to increase or decrease as the temperature increases? Why?
[Hint: The forward reaction is endothermic as energy is required to break into .]

