Chemical Equilibrium and Its Characteristics
Important Questions on Chemical Equilibrium and Its Characteristics
The equilibrium concentration of for the reversible reaction can be evaluated by the expression:
For the given reaction at constant pressure,
Then, the correct relation between initial density and final density of the system is:
In the following reaction, which started only with ,
, mole fraction of is found to at a total pressure of at equilibrium. The mole fraction of at equilibrium is:
In chemical reaction the system will be known in equilibrium when,
For the reaction: . The backward reaction at constant temperature is favoured by:
In the reaction a graph in plotted to show the variation of rate of forward and backward reactions against time. Which of the following is correct?
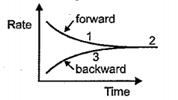
Which of the following is incorrect about the chemical equilibrium?
Rate of reaction curve for equilibrium can be like:
Rate of reaction curve for equilibrium can be like:
Which of the following statement is incorrect:
Which of the following reactions can reach equilibrium in open vessel?
What is the effect of on the solubility of (neglect hydrolysis of ion)?

