Comparison of Alkali Metals and Halogens
Important Questions on Comparison of Alkali Metals and Halogens
The table below does not represent hydrogen. Some elements are given their own symbol and position in the periodic table while others are shown with a letter.
| 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| D | |||||||
| A | E | ||||||
| B | C | G | L |
Draw the electron dot structure for the compound formed between and
The table below does not represent hydrogen. Some elements are given their own symbol and position in the periodic table while others are shown with a letter.
| IA | IIA | IIIA | IVA | VA | VIA | VIIA | O |
| 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Li | D | O | J | Ne | |||
| A | Mg | E | Si | H | K | ||
| B | C | F | G | L |
In the compound between and what type of bond will be formed?
The elements of one short of the periodic table are given below in order from left to right:
Which one of the above elements belongs to the halogen series?
The elements of one short of the periodic table are given below in order from left to right:
Place the three elements fluorine, beryllium, and nitrogen in the order of increasing electronegativity.
The elements of one short of the periodic table are given below in order from left to right:
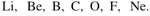
One element of this period is missing. Which is the missing element, and where should it be placed?
The elements of one short of the periodic table are given below in order from left to right:
To which period do these elements belong?
M is a metal above hydrogen in the activity series and its oxide has the formula . This oxide when dissolved in water forms the corresponding hydroxide which is a good conductor of electricity. Name the group to which M belongs.
In the periodic table alkali metals are placed in the group:
The question refers to the elements of the Periodic Table with atomic numbers and . Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.
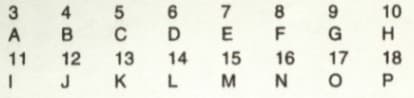
Which of these is an alkali metal?
The question refers to the elements of the Periodic Table with atomic numbers to . Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.

Which of these is a halogen?
Elements of group and elements of group both have valency . Explain.
Name the metal present in Period , Group of the Periodic Table.
Give reason for the following:
Alkali metals are good reducing agents.

