Manufacture of Sulphuric Acid (Contact Process)
Important Questions on Manufacture of Sulphuric Acid (Contact Process)
In the manufacture of sulphuric acid by contact process give the equations for the conversion of sulphur trioxide to sulphuric acid.
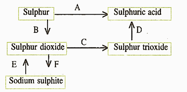
(d) Write the equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step .
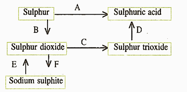
(c) What type of substance will liberate sulphur dioxide from sodium sulphite in step ?
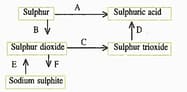
(b) In the Contact process for the manufacture of sulphuric acid, sulphur trioxide is not converted to sulphuric acid by reacting it with water. Instead, a two steps procedure is used. Write the equations for the two steps involved in .
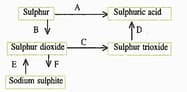
(a) Name the catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide in step C.
State the conditions required for the following reactions to take place the conversion of sulphur dioxide to sulphur trioxide.
Give a balanced equation for the Manufacture of sulphuric acid by the contact process.
Copy and complete the following table relating to an important industrial process and its final output.
| Name of process | Inputs | Catalyst | The equation for catalysed reaction output |
| Contact process | Sulphur dioxide + oxygen |
Why the impurity of arsenic oxide must be removed before passing the mixture of and air through the catalytic chamber?
Sulphuric acid is manufactured by the Contact process. (e) Name a gas that can oxidise sulphur.
Sulphuric acid is manufactured by the Contact process. (e) Name the chemical used to dissolve and also name the product formed. Give all the main reactions of this process.
Sulphuric acid is manufactured by the Contact process. (d) Why is not obtained by directly reacting with water?
Sulphuric acid is manufactured by the Contact process. (c) Name the catalyst used.
Sulphuric acid is manufactured by the Contact process. (b) Give the conditions for the oxidation of .
Sulphuric acid is manufactured by Contact process. (a) Give two balanced equations to obtain in this process.

