Allotropes of Carbon
Important Questions on Allotropes of Carbon
Thermodynamically the most stable form of carbon is
Graphite is used in nuclear reactor:
Which of the following is used in making printer's ink, black varnish and shoe polish?
Graphite and diamond will behave differently in which of the following reactions?
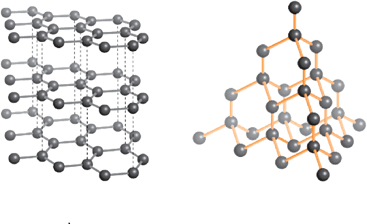
One of the most fascinating developments in modern chemistry has been the synthesis of Buckminster fullerene, . Identify the correct options about .
Select the correct statement(s).
Consider a prototypical fullerene, .
Let Number of -membered rings
Number of -membered rings
Number of -bonds in
Find the value of
How many of the following statements are correct regarding allotropes of carbon
Graphite is not a good conductor of electricity in the perpendicular direction of layers at ordinary temperatures.
Coke is an impure form of carbon.
Anthracite is the purest form of carbon.
Buckminster fullerene contains five-membered rings and six-membered rings.
Diamond is a good conductor of heat.
Graphite is diamagnetic in nature.
Graphite is thermodynamically more stable than diamond.
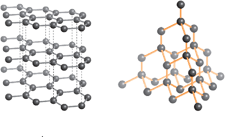
Given the order of density: Diamond Graphite Fullerene ; choose the correct order for bond length.
(Consider the larger bond length if there are two different bond lengths)
Relatively most inert form of carbon is:
Which is not the property of diamond?
Graphite is used as a lubricant. Explain?
Graphite is a soft solid lubricant extremely difficult to melt. The reason for this anomalous behaviour is
that graphite

