Preparation of Alcohols
Preparation of Alcohols: Overview
This Topic covers sub-topics such as Methods of Preparation of Alcohols, Mechanism of Acid Catalysed Hydration of Alkenes, Preparation of Alcohols by Reduction of Aldehydes and Ketones and, Preparation of Alcohols By Acid Catalysed Hydration of Alkenes
Important Questions on Preparation of Alcohols
Match the conversions with the reagents that are used in the process:
| i. | Phenol to benzoquinone | a. | H2O/H+ |
| ii. | Propanone to 2-methylpropan-2-ol | b. | Na2Cr2O7/H2SO4 |
| iii. | Propene to propan-2-ol | c. | CH3MgBr/H2O |
An organic compound A on reduction forms compound B . B reacts with HBr to form the compound C. C with Mg forms Grignard reagent D which reacts with A to form a product which on hydrolysis gives E. Identify A to E.
In the following sequence of reactions,
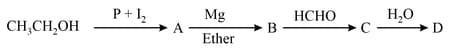
The compound D is –
Between and, which cannot be produced by the reduction of either an alcohol or an aldehyde? Why?
What happens when Pent-1-ene is reacts with dilute sulphuric acid.
Reaction of 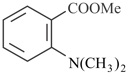 with two equivalent of followed by acidic hydrolysis forms compound, Dehydration of forms compound. Answer the following question on the basis of above write up.
with two equivalent of followed by acidic hydrolysis forms compound, Dehydration of forms compound. Answer the following question on the basis of above write up.
What type of alcoholic group is present in compound (P)?
Reaction of 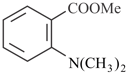 with two equivalent of followed by acidic hydrolysis forms compound, Dehydration of forms compound. Answer the following question on the basis of above write up.
with two equivalent of followed by acidic hydrolysis forms compound, Dehydration of forms compound. Answer the following question on the basis of above write up.
Which simplest product is formed by reductive ozonolysis of compound ?
How can one degree alcohol be converted to two degree alcohol without changing the number of carbon?
What is the product formed in the following reaction?
An organic compound on reaction with followed by acid treatment gives compound .The compound, on reaction with gives compound, . Identify compound and .
Identify the catalyst in the hydration of alkenes to produce alcohols.
The product 'A' of the following reaction is
The major product formed in the following reaction is
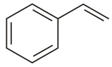
In which of the following the adduct obtained gives Propyl alcohol on hydrolysis?
In which of the following reactions -Phenylbutan--ol cannot be prepared?
Explain the mechanism of acid catalysed hydration of alkenes to form alcohols.
When equivalence ratio of the gases and are heated to at atm in the presence of catalyst, methanol is formed. Here, the gases and are _____ and _____ respectively.
Which of the following compound gives -Ethylpentan--ol by the action of ethyl magnesium iodide followed by acid hydrolysis?
Which of the following alcohols cannot be prepared by reduction of carbonyl compounds?
Which one of these reactions will produce a primary alcohol?
