Reactions of Phenol
Reactions of Phenol: Overview
This topic covers concepts, such as, Chemical Properties of Phenols,Acidic Character of Phenols,Effect of Substituents on the Acidity of Phenol etc.
Important Questions on Reactions of Phenol
Which of the following statements are true?
(i) Phenol is a stronger acid than alcohol.
(ii) Alcohols are comparatively more soluble in water than the corresponding hydrocarbons.
When sodium phenoxide is heated with carbon dioxide under pressure, sodium salicylate is obtained as major product. This on acidification given salicylic acid. This reaction is known as
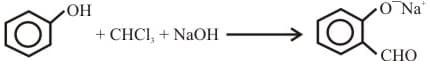
The electrophile involved in the above reaction is
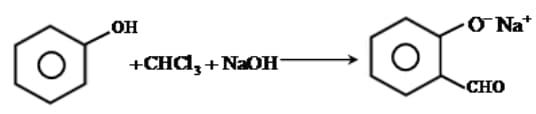
The electrophile involved in the above reaction is
The structure of the compound that gives a tribromo derivative on treatment with bromine water is –
The structure of the compound that gives a tribromo derivative on treatment with bromine water is
Phenol, when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid, gives
A compound when treated with phthalic anhydride in presence of concentrated yields . is used as an acid/base indicator. and are respectively
Increasing order of the following for the electrophilic substitution reaction as:
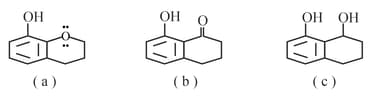
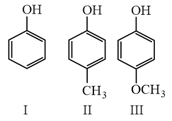
Which of the following compound is most acidic?
Which compound can produce tribromo derivative on reaction with water?
Choose the correct statement(s) is/are regarding Vanilin
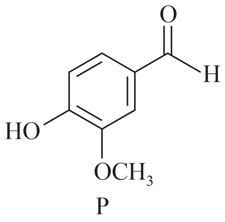
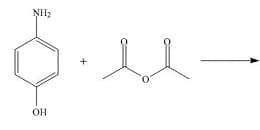
Write down the products for the following reaction:
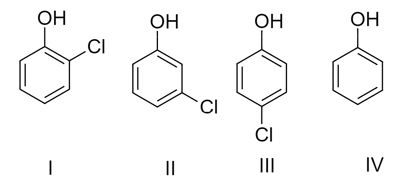
The correct order of acidic nature is
In Kolbes reaction the reacting substances are
Which of the following statements are correct for phenol?
(A) In general, phenol is more acidic than alcohol.
(B) Phenol is used in the production of melamine plastic.
(C) Phenol gives violet colour with neutral ferric chloride solution.
(D) Phenol when heated with acetyl chloride gives phenetole.
in the following reactions are
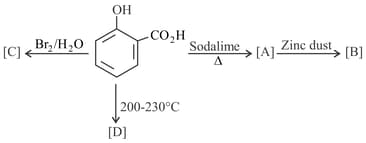
The major product of the following reactions is
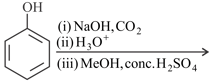
The correct order of acidity of the following is
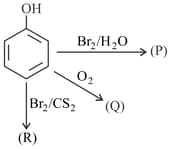
If X = Number of bromine atoms present in(P), Y = Number of pi bonds present in Q and Z = Number of oxygen atoms present in R, then the value of (X + Y + Z) is
