Stereochemical Aspects of Nucleophilic Substitution Reactions
Stereochemical Aspects of Nucleophilic Substitution Reactions: Overview
This topic covers concepts such as Retention of Configuration, Inversion of Configuration, Racemisation, and Stereochemistry of Nucleophilic Substitution Reactions of Alkyl Halides.
Important Questions on Stereochemical Aspects of Nucleophilic Substitution Reactions
If during a reaction, no bond to the stereocentre is broken, than the reaction will proceed with retention of the configuration.
The preservation of integrity of the spatial arrangement of bonds to an asymmetric centre during a chemical reaction is called inversion of configuration.
If during a reaction, the product has the same general configuration of groups around the stereocentre as that of reactant. Such a reaction is said to proceed with:
Explain the retention of configuration with an example.
If the configuration of optically active remains unchanged during the reaction, it is called _____.
In case of optically active alkyl halides reactions are accompainied by _____ which results in zero rotation in polarimeter.
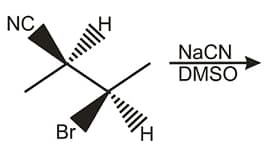
The give reaction
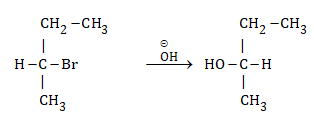 is an example of
is an example of
In reaction, the racemization takes place. It is due to
I : Formation of has proceeded with racemisation
II: Formation of has proceeded with inversion. Select the correct statement:
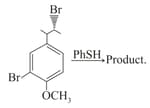
The main product formed in the above reaction is?
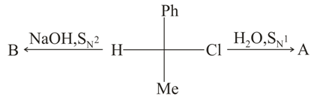
The SN2 reaction involves back-side attack and therefore results in a "Walden Inversion." For which one of the substrates shown would you be able to demonstrate that such back-side attack with "Walden Inversion" has in fact occurred?
Predict the major product of the following reaction.
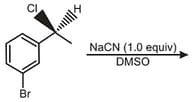
Which of the following is true regarding stereochemistry of product in the following reaction ?