General Introduction to Alkanes
General Introduction to Alkanes: Overview
This topic covers concepts such as Hydrocarbons, Unsaturated Hydrocarbon, Alkanes, Saturated Hydrocarbon, Degree of Carbon Atoms, Nomenclature of Alkanes, Structure of Alkanes, Hybridization in Alkanes, and Bond Angle in Alkanes.
Important Questions on General Introduction to Alkanes
The compound which has one isopropyl group, is –
Alkynes can be represented by the general formula
Give some examples of saturated hydrocarbons.
Which of the following alkanes contain primary, secondary, tertiary and quaternary carbon atoms together?
Define saturated hydrocarbons and give their general formula.
Identify the correct statement regarding the type of bond between the carbon atoms in the saturated hydrocarbons.
Unsaturated hydrocarbons are compounds that have a single bond between two adjacent atoms of carbon.
In which of the following compounds double bonds alternate between carbon atoms of the benzene ring?
Which of the following elements are present in compounds known as hydrocarbons?
How many bonds are present in ethane molecule?
Name the type of hydrocarbon that is used as lubricant.
Write the IUPAC name for the following molecule:
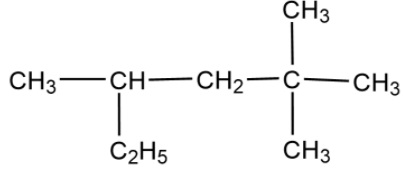
Trimethylhexane
Trimethylhexane
Trimethylpentane
Enter your correct answer as .
Give an appropriate term for the following: Compounds containing carbon and hydrogen only. (Hydrocarbons/Halogens)
Write the formula for 1,3-Dichloropropane.
Write the formula for isopentane.
Write the IUPAC name and structural formula of .
Write the IUPAC name of Isobutane. (2-Ethylbutane/ 2-methylpropane)
Name the most important natural source of hydrocarbon. (Petroleum / Water / Soil)
Which of the following IUPAC name is/are correct?
Select the correct statements about the following compound.
