Physical Properties of Alkanes
Physical Properties of Alkanes: Overview
This topic covers concepts, such as, Physical Properties of Alkanes, Boiling Points of Alkanes, Melting Points of Alkanes, Solubility of Alkanes & Density of Alkanes etc.
Important Questions on Physical Properties of Alkanes
Which of the following compounds does not dissolve in conc. even on warming?
The highest boiling point is expected for –
The compound with highest boiling point is –
When cyclohexane is poured on water, it floats, because –
Which of the following alkanes has the highest density.
Alkanes are highly soluble in which of the following solvents:
Constituents of paraffin wax contain solid alkanes ranging from:
Alkanes ranging from are used as______lubricants.
Which of the following alkane has the lowest boiling point and highest melting point?
Which of the following compounds has the highest boiling point?
Which among the following is incorrect statement?
Hydrocarbon that is liquid at room temperature is:
Arrange the following in the decreasing order of their boiling points:
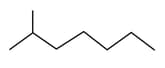
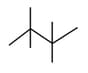
Which of the following is/are correct?
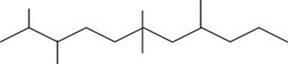
In this molecule
. of carbon atoms
. of carbon atoms
. of carbon atoms
. of carbon atoms
What is the value of ?
Which of the following statements is/are correct?
I. Melting point of alkane increases with increase in number of C atoms and with increase in branching.
II. Boiling point of alkane increases with increase in number of C atoms but with decrease in branching.
III. Cycloalkanes have lower boiling point than normal alkane with same number of C atoms.
IV. Alkenes have lower boiling point than same number of C atoms in alkanes.
When cyclohexane is poured on water, it floats, because :
In laboratory burners, we use
In the series, ethane, ethene and ethyne, the C-H bond energy is
