Preparation Method of Alkenes
Preparation Method of Alkenes: Overview
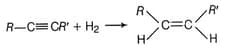
This topic covers concepts, such as Preparation of Alkenes by Dehydration of Alcohols, Preparation of Alkenes by Dehydrohalogenation of Alkyl Halides, Preparation of Alkenes by Partial Reduction of Alkynes by Lindlar Catalyst, etc.
Important Questions on Preparation Method of Alkenes
Which of the following compound is formed on acid dehydration of ethanol?
2-hexyne gives trans-2-hexene on treatment with –
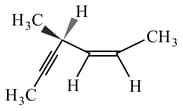
Hydrogenation of the adjoining compound in the presence of poisoned palladium catalyst gives –
The reagent(s) for the following conversion,
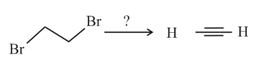
is/are
Cyclohexene is best prepared from cyclohexanol by which of the following?
A solution of of -dibromopropane in upon heating with excess produces of an unsaturated compound The yield () of is closest to [Atomic weight of Br is ]
The compound that undergoes dehydration most readily is
From which one of the following both ethylene and acetylene could be prepared in a single step reaction?
The product obtained on elimination of bromine from 2-bromobutane is:
Which of the following methods cannot be considered suitable for preparing ether?
The best reagent for the preparation of cyclohexene from cyclohexanol is
Which reagent(s) is/are used to carry out the following conversion?
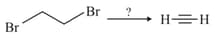
When is heated with what will be the product obtained?
Dehydration of alcohols by concentrated takes place according to the following steps:

The slowest and fastest steps in the above reaction are:
In petrochemical industry, alcohols are directly converted to gasoline by passing over heated
Optically active 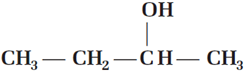 was found to have lost its optical activity after standing in water containing a few drops of acids, mainly due to the formation of
was found to have lost its optical activity after standing in water containing a few drops of acids, mainly due to the formation of
The reagent X used for the following reaction is
Compound 'X' on Heating with Zn dust gives compound 'Y' which on treatment with followed by reaction with Zn dust gives propionaldehyde. The structure of 'X' is
The product that should be formed in the reaction is:
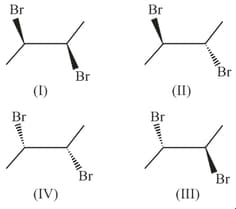
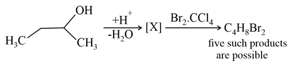
How many structures of is possible?