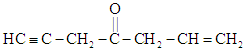Tetravalency of Carbon
Tetravalency of Carbon: Overview
This topic consists of various concepts like Tetravalency of Carbon,Shapes of Organic Compounds,Role of Hybridisation in Shapes of Organic Compounds, etc.
Important Questions on Tetravalency of Carbon
In which of the following molecules, all atoms are not coplanar?
Which one of the following does not have hybridised carbon ?
In the compound,  , the bond is of the type –
, the bond is of the type –
The hybridisation of carbon atoms in the C-C single bond of is:
Which of the following represent the given mode of hybridisation from left to right?
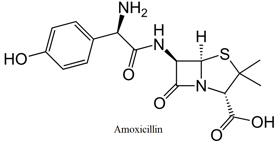
In the molecule given above how many atoms are unhybridised.
The ratio of and hybrid orbitals in the following molecule is
Write the maximum number of atoms having same hybridisation in the following structure
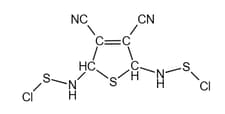
The carbon atoms in are
The molecule which contains and bond in it is
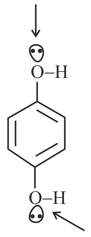
The hybridisation of the above oxygen atoms of -OH group is
How does hybridization affect the electronegativity?
Arrange the following bonds in decreasing order of bond energy :
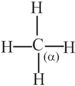
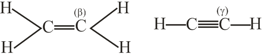
The bond between carbon atom and carbon atom in compound
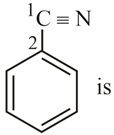
The cylindrical shape of alkynes is due to
Describe shape of alkene.
Describe the shape of alkanes.
How many carbon atoms show tetrahedral geometry in the given structure?
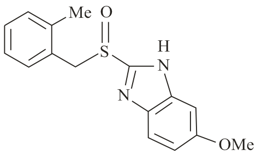
Which of the following structure contains sp-hybridized carbon atoms?
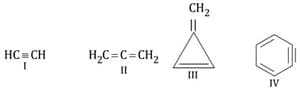
The number of hybridized carbon atoms in