Types of Organic Reactions
Types of Organic Reactions: Overview
This topic covers concepts, such as, Types of Organic Reactions, Substitution Reactions, Markownikoff's Rule for Electrophilic Addition Reaction & Stereochemistry of Elimination Reaction etc.
Important Questions on Types of Organic Reactions
and reactions differ from each other because of:
The reaction of –
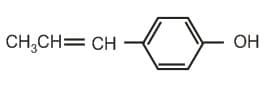
with HBr gives
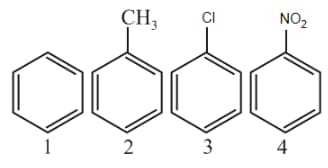
Identify the correct order of reactivity in electrophilic substitution reactions of the following compounds.
Write notes on Markownikoff's rule.
The major product of the following reaction is _________
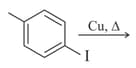
Which of the following types of reactions takes place when n- butane changes to iso-butane in the presence of aluminium chloride?
The reaction of ethene with hydrogen in the presence of nickel catalyst to form ethane is an example of substitution reaction.
Which of the following types of reaction takes place when ethane reacts with chlorine in the presence of sunlight?
The reaction, 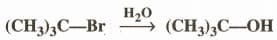 is
is
Which of the following statements are correct?
Which of the following is a elimination reaction?
Pick out the correct reactions.
What is the major product of the reaction?
Which of the following compound is most rapidly hydrolysed by mechanism?
The product Z of the following reaction is
Hypochlorous acid (HOCl) is unstable therefore water is used for reaction. According to Markownikov addition, is attached with that carbon containing less number of hydrogen, while the reverse is Anti - Markownikov addition.
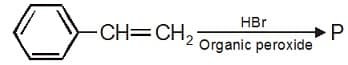
Product P may be-
Which one of the following compounds gives , and mechanisms?
Arrange the following in order of increasing rate of nucleophilic substitution
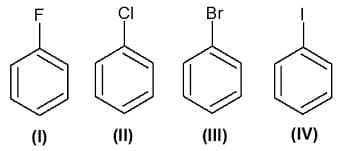
The reagent(s) for the following conversion,
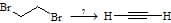
is & are
The intermediate during the addition of HCl to propene in the presence of peroxide would be

