Physical Properties of Amines
Physical Properties of Amines: Overview
In this topic, we will study physical properties of amines, including the primary and secondary amines along with intermolecular hydrogen bonding. It also contains comparison of boiling point of amines, alcohols and alkanes of similar molecular masses.
Important Questions on Physical Properties of Amines
Arrange the following in decreasing order of their boiling points:
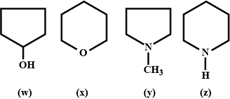
The correct order of boiling points of isomeric amines is
Select the correct statement about amines from the follwoing.
is soluble in which of the following compound?
Among the following molecules with the same molecular weight, which one has the highest boiling point?
Increasing order of basic nature of , and 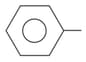 is :
is :
Explain briefly about the odour of amines?
Explain briefly about the colour of aryl amines?
Arrange the following compounds in the decreasing order of their basic nature in gaseous phase. Ammonia, N - methylethanamine, propan - I - amine and N, N - dimethylmethanamine
Arrange the following amines in the increasing order of their values
aniline, 4 - methoxyaniline and 4 - nitroaniline
Arrange the following amines in the increasing order of their values
Phenylmethylamine, 2 - Aminotoluene and 2 - Fluoroaniline
Arrange the following compounds in the decreasing order of their boiling points
Ethyl alcohol, ethylamine and diethylamineArrange the following compounds in the decreasing order of their boiling points
Ethylamine, n-propylamine, n-butylamineArrange the following compounds in the decreasing order of their boiling points
Ethane, ethylamine, and ethyl alcohol
Arrange the following compounds in the decreasing order of their solubility in water
n-butane, n-butyl alcohol and n-butylamineGive plausible explanation for the statements. Butan - 1 - amine has higher boiling point than N - Ethylethanamine.
Give plausible explanation for the statements.-Butan - 1 - ol is more soluble in water than Butan - 1 - amine.
Give plausible explanation for the statement-Ethylamine is soluble in water whereas aniline is not.
Arrange the following compounds in the decreasing order of their solubility in water
ethylamine, n-propylamine and n-butylamine
Arrange the following compounds in the decreasing order of their solubility in water
ethylamine, diethylamine and triethylamine
