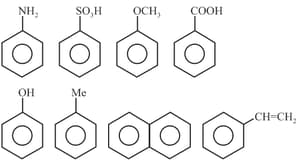
Application of Electronic Effect
Application of Electronic Effect: Overview
This topic covers concepts, such as, Bond Parameters, Bond Order, Effect of Backbonding on Basic Strength of Compounds & Steric Inhibition of Resonance etc.
Important Questions on Application of Electronic Effect
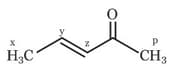
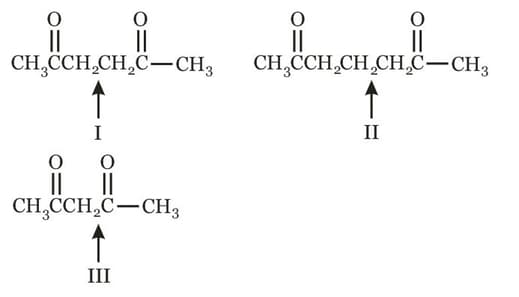
The abstraction of proton will be fastest from which carbon in the following compound?
Which of the following statements would be true about the below compound?
Which of the following has longest C-O bond:
Rank the following compounds in order of decreasing acidity of the indicated hydrogen :
The increasing order of basic strengths in their aqueous solutions is:
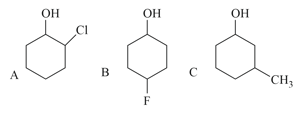
Which of the following compound do not give the protonation reaction readily?
Amongst the following, the most basic compound is
What is the correct order of acidity of the protons marked in the given compounds?
Given below are two statements, one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : Order of acidic nature of the following compounds is .
Reason R: Fluoro is a stronger electron withdrawing group than Chloro group.
In the light of the above statements, choose the correct answer from the options given below :
Correct statements for the given reaction are:
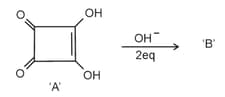
A. Compound ‘’ is aromatic
B. The completion of above reaction is very slow
C. ‘’ shows tautomerism
D. The bond lengths of in compound are found to be same
Choose the correct answer from the options given below.
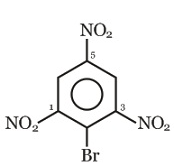
The strongest acid from the following is
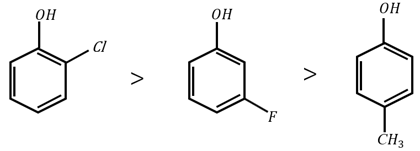
The correct order for acidity of the following hydroxyl compound is
(A)
(B)
(C) 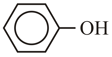
(D) 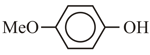
(E) 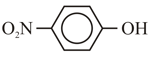
Choose the correct answer from the options given below:
The descending order of acidity for the following carboxylic acid is-
(A)
(B)
(C)
(D)
(E)
Choose the correct answer from the options given below:
Assertion(A): Acidic nature follows the order
Reason: is better electron withdrawing group than .
In which of the following pair, the indicated bond has the greatest strength.
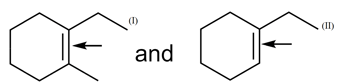
Arrange the acidic order of the following compounds in decreasing order
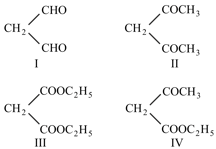
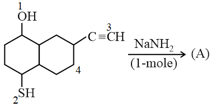
The hydrogen removed in the Product at the position
Arrange marked atom in decreasing order of acidic strength
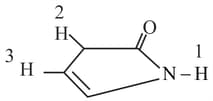
Which of the following is most acidic?
Amongst the following, the total number of the compounds soluble in aqueous is: