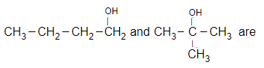Structural Isomerism
Structural Isomerism: Overview
This topic covers concepts, such as, Structural Isomerism, Chain Isomerism, Acid-catalyzed Enolisation & Relative Stabilities of Keto and Enol Tautomers etc.
Important Questions on Structural Isomerism
The compound C2H5OC2H5 and CH3OCH2CH2CH3 are
Molecular formula can have (including stereoisomer):
Phenol and benzyl alcohol are
How many structural formula are possible when one of the hydrogen is replaced by a chlorine atom in anthracene?
C7H7Cl shows how many benzenoid aromatic isomers?
The compounds are:
The enolic form of acetone contains
The total number of structural isomers of C6H14 is:
An isomer of ethanol is:
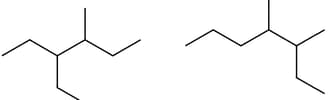
Relation between the above compounds is
The minimum number of carbon atoms that should be present in an organic compound to be able to show position isomerism is :
Are tautomers called functional isomers?
Which of the following are not chain isomers?
The number of amides possible from molecular formula is
The number of structural isomers possible from the molecular formula is
Positional isomers differ in
Which of the following pair represents chain isomers?
Out of the different structural isomers possible with molecular formula , how many are tertiary alkyl bromides?
Which of the following statements is false regarding the enolization of
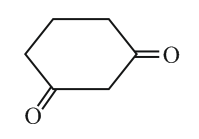
It can form two types of enolic forms of equal stability.
It can form only two types of enolic forms of different stability.
It can form more than two types of enolic forms of different stability.
It can form only one type enolic form.