Adsorption
Adsorption: Overview
This Topic covers sub-topics such as Adsorption, Van der Waals Forces, Adsorption Isotherms, Freundlich's Adsorption Isotherm, Adsorbent, Desorption, Physical Adsorption, Adsorbate, Sorption, Mechanism of Adsorption and, Chemical Adsorption
Important Questions on Adsorption
Among physisorption and chemisorption, which type of a adsorption has a higher enthalpy of adsorption?
Four gases, and have critical temperatures and respectively
For their adsorption on a fixed amount of charcoal, the correct order is :
Which of the following represents the Freundlich adsorption isotherms?
(A)
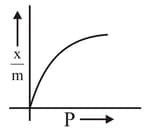
(B)
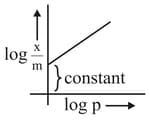
(C) 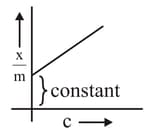
(D) 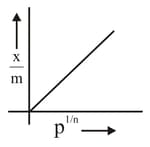
Choose the correct answer from the options given below:
Critical temperature of gases A, B, C and D are given as . Find the correct order of adsorption of gases from the options given below.
Give initial for true statement and for false statement respectively.
I. Van der Waal forces are responsible for the formation of dry ice.
II. Dipole-induced dipole forces are present the clathrate compounds
III. London's dispersion forces are responsible for liquefication of Noble gases.
IV. For dipole-dipole interactions (E = energy and r = intermolecular distance)
Among the given gases, the gas with the highest van der Waal's force of attraction is
The wrong statement about Freundlich isotherm is/are
A) It can be represented at high pressure as
B) The slope of the straight line of '' Vs ', is ''
C) It explains the adsorption behaviour in an approximate manner
D) It holds good in the pressure range to
Which one of the following is not true with regard to physisorption?
The correct option(s) related to adsorption processes is(are)
When hydrogen gets occluded on palladium, the conductivity of the metal
Which is Freundlich Adsorption isotherm?
A container contains of solution of cyclobutane in ether. A piece of charcoal is dipped in the solution. Molecules of cylobutane get adsorbed on the surface of charcoal and form a monolayer of cyclobutane. The molarity of resulting solution decreases to $1 \mathrm{M}$. If surface area available for adsorption on charcoal is , then find distance between two adjacent carbon atoms (in pm) in a cyclobutene molecule.
(Assume : Shape of cyclobutane molecules as a perfect square & ).
Write a short note on adsorption. How does adsorption differ from absorption? What are different types of adsorption?
The volume of nitrogen gas (measured at )required to cover a sample of silica gel with a monomolecular layer is of gel. Calculate the surface area per gram of the gel if each nitrogen molecule occupies .
In an adsorption experiment, a graph between versus was found to be linear with a slope of . The intercept of the was found to be . Calculate the amount of gas adsorbed per gram of the charcoal under a pressure of atmosphere.
A one-litre vessel contained a gas at , of charcoal was introduced into it. The pressure of the gas fell down from to . Calculate the volume of the gas (at S.T.P) adsorbed per gram of charcoal. Density of charcoal sample used was .
Explain the following giving reasons: Rate of physical adsorption decreases with rise of temperature.
Explain how the phenomenon of adsorption finds application in Heterogeneous catalysis.
