Hydrogen Atom Spectrum
Hydrogen Atom Spectrum: Overview
This topic covers concepts, such as, Hydrogen Emission Spectrum, Explanation of Line Spectrum of Hydrogen, Spectral Lines, Rydberg's Equation, Lyman Series, Balmar Series, Paschen Series, Brackett Series, Pfund Series & Humphrey Series etc.
Important Questions on Hydrogen Atom Spectrum
The spectrum of He is expected to be similar to that
In the Balmer series of atomic spectra of hydrogen, there is spectral line having wavelength . Calculate the number of higher orbit from which the electron drops to generate this line.
Which of the following is the value of the Rydberg constant for hydrogen?
Among the following, which transition in the Hydrogen spectrum would have the same wavelength as Balmer transition, to in spectrum?
If the wavelength of the first line in Balmer series is , then the wavelengths of its second line and limiting line respectively are
Which energy level transition among the following will have the least wavelength?
The electron in a hydrogen atom jump on absorbing of energy would jump to orbit
The shortest wavelength in hydrogen spectrum is approximately ______
Which of the following series of transitions in the spectrum of hydrogen atom falls in visible region?
Calculate the shell number of excite state of -atom in terms of wavelength , when the electron excites to the ground level.
In the Balmer series of atomic spectra of hydrogen, there is spectral line having wavelength . Calculate the number of higher orbit from which the electron drops to generate this line.
What transition in the spectrum would have the same wavelength as the first Lyman transition of hydrogen ?
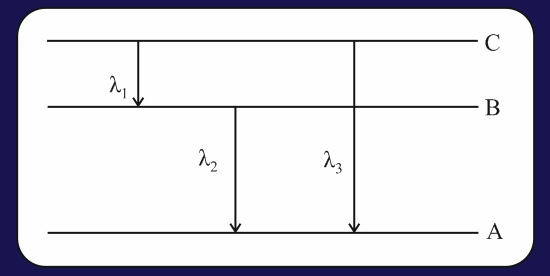
Energy levels of of a certain atom correspond to the increasing value of energy, i.e.. If
The values of in the Rydberg equation for Humphrey series is?
In Pfund series what is the value of n1 and n2 in the following equation:
The region in the electromagnetic spectrum where the Balmer series lines appear is:
The region in the electromagnetic spectrum where the Balmer series lines appear is:
The series of lines present in the visible region of the hydrogen spectrum is
Match the entries of with appropriate entries of and choose the correct option out of the four options given at the end of each question.
| Column I | Column II | ||
| A | Lyman series | p | Infrared region |
| B | Paschen series | q | Ultraviolet region |
| C | Pfund series | r | Visible region |
| D | Balmer series | s | Infrared region |
If is the wavelength of the line for jump of the electron from that of the line for jump from , then wavelength of the line for the jump from orbit will be
