Particle Nature of Electromagnetic Radiations
Particle Nature of Electromagnetic Radiations: Overview
This topic covers concepts, such as, Black Body Radiation, Properties of Black Body, Evidences of Particle Nature of Electromagnetic Radiations & Wavelength-Intensity Relationship for Black Body etc.
Important Questions on Particle Nature of Electromagnetic Radiations
The energies of two radiations are and , respectively. The relation between their wavelength i.e., will be:
The value of Planck’s constant is The velocity of light is Which value is closest to the wavelength in meters of a quantum of light with frequency of
The number of incorrect statement/s about the black body from the following is______
(A) Emit or absorb energy in the form of electromagnetic radiation.
(B) Frequency distribution of the emitted radiation depends on temperature.
(C) At a given temperature, intensity vs frequency curve passes through a maximum value.
(D) The maximum of the intensity vs frequency curve is at a higher frequency at higher temperature compared to that at lower temperature.
Frequency of electromagnetic wave is . Identify the not possible values of total energy for this frequency.
What is the number of photons of light with a wavelength of that provide of energy?
A perfectly black body
The approximate value of Planck's constant is equal to _____.
What is the difference between a photon and a quantum ?
Planck’s constant has the units of
Which of the following transitions will have minimum wavelength?
mole of photons, each of frequency would have approximately a total energy of
mole of photons, each of frequency would have approximately a total energy of
Chose options is nearly to black body.
A sodium lamp radiates energy uniformly in all directions.The energy per photon in (eV) associated with the sodium light if the wavelength of the sodium light is .Given that the lamp is located at the center of a large sphere that absorbs all the sodium light which is incident on it.
If the wavelength of light is 4000 pm and energy 1J then determine the number of photons of light emmitted.
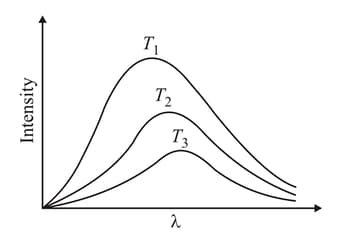
What is the correct order of temperatures for above black body radiation curve-graph?
The number of photons radiated per second from a source emitting a wavelength of ?
Take
The energy difference between the ground state of an atom and its excited state is , calculate the wavelength of the photon required to induce the transition.
If the wavelength of an electron moving with velocity of 2.05 x 107 ms-1 then value of m is
A certain dye absorbs light of and then fluorescence light of . Assuming that under given conditions of the absorbed energy is re-emitted out as fluorescence, calculate the ratio of quanta emitted out to the no. of quanta absorbed.
