Integrated Rate Equations - Zero Order Reactions
Integrated Rate Equations - Zero Order Reactions: Overview
This topic covers concepts, such as, Integrated Rate Equation for Zero Order Reactions, Time-Concentration Graph for Zero Order Reactions & Rate-Time Graph for Zero Order Reaction etc.
Important Questions on Integrated Rate Equations - Zero Order Reactions
Which of the following curve represent zero order reaction of products?
Which of the following equations represent integrated rate equation of zero order reaction?
For a zero-order reaction, with the initial reactant concentration , the time for completion of the reaction is:
In the following reaction rate constant is . If we start with of then concentration of and after minutes are :
In the reaction,
The time taken for 75% reaction of P is twice the time taken for 50% reaction of P. The concentration of Q varies with reaction time as shown in the figure. The overall order of the reaction is
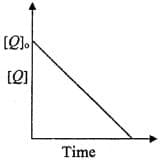
The reaction is a zeroth order reaction. If the initial concentration of is , the half-life is . When the initial concentration of is , the time required to reach its final concentration of will be:
For a zero - order reaction with rate constant k, the slope of the plot reactant concentration against time is
Look at the graph,
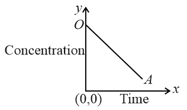
Choose the correct equation from the following which best suited to the above graph
Which of the following curve represent zero order reaction of products?
Which of the following curve represent zero order reaction of products?
For the zero order reaction, ; initial concentration of A is 0.1 M. If [A] = 0.08 M after 10 minutes, then it's half life and completion time are respectively
Which of the following graph represents zero order reaction ?
The unit and value of rate constant and that of rate of reaction are same for:
For zero-order reaction, the integrated rate equation will be:
The rate constant of a zero order reaction is If the concentration of the reactant after min is Then what would be its initial concentration?
The rate constant for a zero order reaction is:
The half-life period for a zero-order reaction , is minutes. How long will it take for completion?
What is the integrated rate law for a zero order reaction?
The time required for 100% completion of a zero order reaction is
The plot between concentration versus time for a zero order reaction is represented by
