Integrated Rate Equations-Zero Order Reactions
Integrated Rate Equations-Zero Order Reactions: Overview
This topic covers concepts, such as, Integrated Rate Equation for Zero Order Reactions, Time-Concentration Graph for Zero Order Reactions & Rate-Time Graph for Zero Order Reaction etc.
Important Questions on Integrated Rate Equations-Zero Order Reactions
The rate constant for a zero order reaction products is . How long it will take for the initial concentration of to fall from to ?
Which of the following curve represent zero order reaction of products?
Which of the following equations represent integrated rate equation of zero order reaction?
For a zero-order reaction, with the initial reactant concentration , the time for completion of the reaction is:
In the following reaction rate constant is . If we start with of then concentration of and after minutes are :
In the reaction,
The time taken for 75% reaction of P is twice the time taken for 50% reaction of P. The concentration of Q varies with reaction time as shown in the figure. The overall order of the reaction is
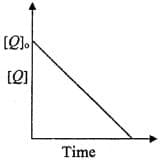
The reaction is a zeroth order reaction. If the initial concentration of is , the half-life is . When the initial concentration of is , the time required to reach its final concentration of will be:
Which of the following curve represent zero order reaction of products?
Which of the following curve represent zero order reaction of products?
For the zero order reaction, ; initial concentration of A is 0.1 M. If [A] = 0.08 M after 10 minutes, then it's half life and completion time are respectively
Which of the following graph represents zero order reaction ?
The unit and value of rate constant and that of rate of reaction are same for:
For zero-order reaction, the integrated rate equation will be:
The rate constant of a zero order reaction is If the concentration of the reactant after min is Then what would be its initial concentration?
The rate constant for a zero order reaction is:
The half-life period for a zero-order reaction , is minutes. How long will it take for completion?
What is the integrated rate law for a zero order reaction?
The time required for 100% completion of a zero order reaction is
The plot between concentration versus time for a zero order reaction is represented by
For zero order reaction, the integrated rate equation is
