Integrated Rate Law for Zero and First Order Reactions
Integrated Rate Law for Zero and First Order Reactions: Overview
This topic covers concepts, such as Zero Order Reactions, Integrated Rate Equation for Zero Order Reactions, Time-Concentration Graph for Zero Order Reactions, First Order Reactions, and Integrated Rate Equation for First Order Reactions.
Important Questions on Integrated Rate Law for Zero and First Order Reactions
A first order reaction is complete in minute. Calculate the time taken for the reaction to be complete:
,
The half-life for decay of radioactive is 5730 years. An archaeological artifact containing wood has only 80% of the activity as found in living trees. The age of the artifact would be:
[Given: log 1.25 = 0.0969]
In a first-order reaction, the concentration of reactant decreases from in. The rate constant of reaction in is
Decomposition of on Gold surface is zero order reaction. Initially few moles of are present in container then which of the following graph is correct?
For a reaction starting with of only. Then calculate the concentration of after respectively are
For the reaction products, the following initial rates were obtained at various given initial concentrations. Determine the half-life period.
Select the correct statements out of I, II and III for a zero order reaction:
I. Quantity of the product formed is directly proportional to time.
III. If reaction takes place in minutes, reaction will take place in minutes.
III.Larger the initial concentration of the reactant, greater is the half-life period.
In a reaction, the initial concentration of the reactants increases fourfold and the rate becomes eight times its initial value. The order of the reaction is -
The half-life of a radioisotope is hours. If initial mass of the isotope is , the mass (in ) of the remaining undecayed isotope after hours is
The rate-constant of a reaction is . The half-life of this reaction (in s) is
Select the correct statement for a first-order reaction from the following.
The rate constant for the first-order reaction is . How much time will it take to reduce the concentration of the reactant to of initial value?
If 50 % of a reaction occurs in 100 second and 75 % of the reaction occurs in 200 second, the order of this reaction is:
Which of the following curve represent zero order reaction of products?
The inactivation of a viral preparation in a chemical bath is found to be a first order reaction. Calculate the rate constant for the viral inactivation if in the beginning of the virus is inactivated per minute.
Two first-order reactions have half times in the ratio of The ratio of time intervals is:
Here, the time and are time period for and completion.
Which of the following equations represent integrated rate equation of zero order reaction?
Which of the following is incorrect?
For a zero-order reaction, with the initial reactant concentration , the time for completion of the reaction is:
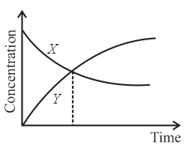
The accompanying figure depicts the change in concentration of species and for the elementary reaction as a function of time. The point of intersection of two curves represents:
