Integrated Rate Law for Zero and First Order Reactions
Integrated Rate Law for Zero and First Order Reactions: Overview
This topic covers concepts such as Integrated Rate Equation for Zero Order Reactions, Half Life Period for Zero Order Reactions, Time-Concentration Graph for Zero Order Reactions, Integrated Rate Equation for First Order Reactions, etc.
Important Questions on Integrated Rate Law for Zero and First Order Reactions
A first order reaction is complete in minute. Calculate the time taken for the reaction to be complete:
,
In a first-order reaction, the concentration of reactant decreases from in. The rate constant of reaction in is
Select whether the following statement is true or false:
The graph that can be plotted between the rate of the reaction and time, for a zero-order reaction, will have a negative slope.
Select whether the following statement/graph is true or false:
The graph between the rate of the reaction and time for a zero-order reaction is:
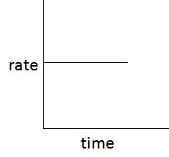
The graph that can be plotted between the rate of the reaction and time for a zero-order reaction will have a slope of _____ value. (negative/ zero/ positive)
Explain the graph that can be plotted between the rate of the reaction and time for a zero-order reaction.
For a zero-order reaction, with the initial reactant concentration , the time for completion of the reaction is:
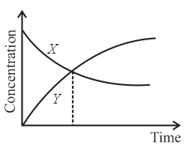
The accompanying figure depicts the change in concentration of species and for the elementary reaction as a function of time. The point of intersection of two curves represents:
The order of a reaction for which a linear line (with a negative slope) is obtained in a graph of vs is:
Two-third life for a first-order reaction is minutes. The value of its velocity constant is nearly
Consider a reaction that is first order in both directions
Initially only is present, and its concentration is Assume and are the concentrations of at time and at equilibrium, respectively. The time at which is:
For a first order reaction , the rate constant is . If the initial concentration of is , the concentration of at any time is given by the expression:
The time taken for the completion of three-fourths of a first-order reaction is
The half-life period for a zero order reaction is equal to:
Show that, In a first order reaction, time required for completion of is _____ times of half life. ().
The half-life period of a zero order reaction product is given by:
A first order reaction has reaction constant, . The half-life of the reaction in seconds is: _____
The half life of first order reaction is hours. Time take (in hours) for the concentration of reactant to decrease from to is:
A first order reaction takes minutes for decomposition. Calculate half life?
What is the mathematical relation between half life of zero order reaction and its rate constant.
