Second Law of Thermodynamics
Second Law of Thermodynamics: Overview
This topic covers concepts, such as, Second Law of Thermodynamics, Scope of Second Law of Thermodynamics, Carnot Cycle & Idea behind Carnot Cycle etc.
Important Questions on Second Law of Thermodynamics
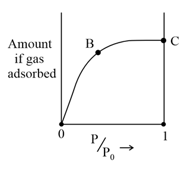
The adsorption of a gas at the boiling point of the gas follows the isotherm shown in the figure. Identify the correct thermodynamic properties at point
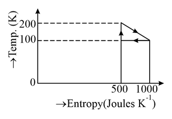
A heat engine absorbs heat at temperature and heat at temperature . Work done by the engine is . This data
The unit of is
What does entropy measure?
The change in entropy at equilibrium is
Which of the following statement is NOT correct, according to second law of thermodynamics?
Enthalpy (H) for the reaction, and emf of the cell was of the cell is?
In Carnot cycle, an ideal gas is taken through 4 reversible steps as shown in the diagram.
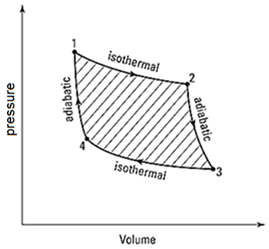
Which of the following statement(s) correct?
(i) ΔU is positive in the step between points 2 and 3.
(ii) ΔU is positive in the step between points 4 and 1.
(iii) ΔU is negative in the step between points 2 and 3.
(iv) Temperature at point 4 is higher than point 2.
Find the amount of heat energy supplied to a Carnot engine from the source in each cycle if the engine is working between and and it has a work output of per cycle.
The difference in H and U from combustion of solid benzoic acid at 300K is equal to :
A piece of ice kept at room temperature melts on its own . This reaction is governed by which law?
Identify the correct statement regarding a spontaneous process:
Consider entropy (S) as a thermodynamic parameter, the criterion for the spontaneity of any process is
What percentage is of for a efficiency of a heat engine? is the temperature of sink and is the temperature of heat reservoir.
Which of the following is the most effective way to increase the efficiency of the carnot engine?
For a perfectly crystalline solid Cp,m = aT3 , where a is constant. If Cp,m is at 10 K, molar entropy at 20 K is :
A piece of ice kept at room temperature melts of its own. This reaction is governed by which law?
The entropy change involved in the isothermal reversible expansion of 2 moles of an ideal gas from a volume of 10 to a volume of 100 is
The efficiency of a Carnot engine is and temperature of sink is . If temperature of source is kept constant and its efficiency raised to , then the required temperature of sink will be:
The efficiency of the reversible cycle in the given figure is